研究成果发表于JACS
更新时间:2018-05-29
Xi-Meng Chen,†,⊥ Nana Ma,†,⊥ Qian-Fan Zhang,‡ Jin Wang,† Xiaoge Feng†, Changgeng Wei,† Lai-Sheng Wang,*,‡ Jie Zhang,*, † and Xuenian Chen*,†
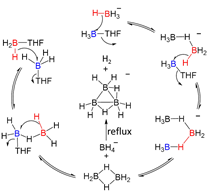
Boron compounds are well-known electrophiles. Much less known are their nucleophilic properties. By recognition of the nucleophilicity of the B-H bond, the formation mechanism of octahydrotriborate (B3H8−) was elucidated on the bases of both experimental and computational investigations. Two possible routes from the reaction of BH4− and THF•BH3 to B3H8− were proposed, both involving the B2H6 and BH4− intermediates. The two pathways consist of a set of complicated intermediates, which can convert to each other reversibly at room temperature and can be represented by a reaction circle. Only under reflux can the B2H6 and BH4− intermediates be converted to B2H5− and BH3(H2) via a high energy barrier, from which H2 elimination occurs to yield the B3H8− final product. The formation of B2H6 from THF•BH3 by nucleophilic substitution of the B−H bond was captured and identified, and the reaction of B2H6 with BH4– to produce B3H8− was confirmed experimentally. On the bases of the two formation mechanisms of B3H8–, we have developed a facile synthetic method for MB3H8 (M = Li and Na) in high yields by directly reacting the corresponding MBH4 salts with THF•BH3. In the new synthetic method for MB3H8, no electron carriers are needed, allowing convenient preparation of MB3H8 in large scales and paving the way for their wide applications.
该论文第一作者为陈学年老师的博士生陈西孟同学和麻娜娜老师,第一单位为河南师范大学。原文链接为https://pubs.acs.org/doi/10.1021/jacs.8b03785